In today’s age of data-driven biology, the challenge isn’t how to generate biological data-it’s how to make sense of it. Technologies like next-generation sequencing, mass spectrometry, and microarrays have ushered in the era of omics: large-scale data on genes (genomics), transcripts (transcriptomics), proteins (proteomics), metabolites (metabolomics), and epigenetic modifications (epigenomics). The result is complex, high-dimensional datasets that traditional statistical methods struggle to interpret. This is where artificial intelligence (AI) steps in-not as a tool of convenience, but as a necessity.
AI is transforming modern biology by helping researchers uncover patterns, predict outcomes, and make discoveries at a speed and scale that were previously unimaginable. In this article, we’ll explore how AI-including machine learning (ML), deep learning (DL), and natural language processing (NLP)-is revolutionizing omics data analysis, and why students and researchers alike should pay attention.
Why AI Matters in Omics Data Analysis
AI refers to systems that can mimic human intelligence by learning from data and making informed decisions. In the context of omics, AI offers a way to manage, analyze, and interpret complex biological data that is too voluminous and intricate for manual analysis. For example, a single RNA-seq experiment may produce expression values for 20,000 genes across thousands of cells. Extracting biological insights from such data requires intelligent algorithms capable of filtering noise, learning structure, and identifying meaningful associations.
AI excels in handling these challenges. It allows researchers to classify disease subtypes, identify biomarkers, predict patient outcomes, and integrate data from multiple omics layers. The result is not just faster science, but more personalized and predictive approaches to medicine and research.
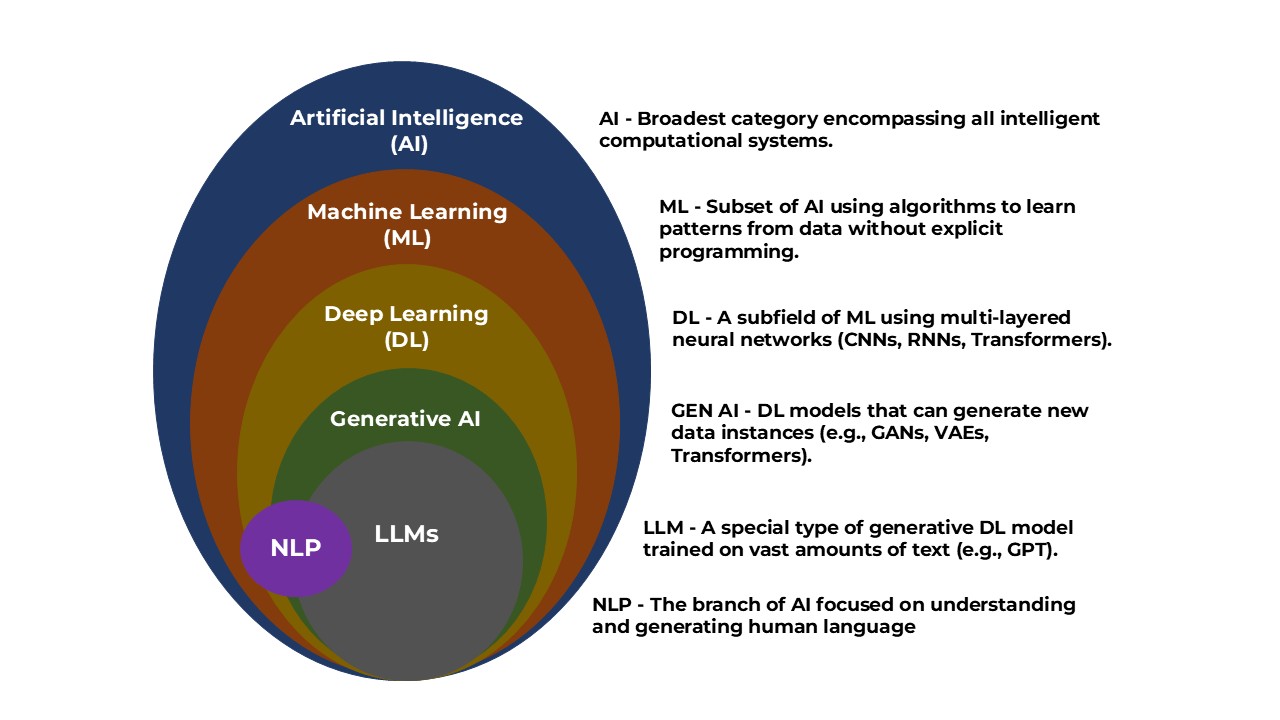
Artificial Intelligence in Omics
Artificial Intelligence (AI) is the overarching field that enables the development of intelligent tools for interpreting biological complexity. In omics, AI facilitates large-scale pattern recognition across genomics, transcriptomics, and proteomics datasets. It supports tasks like hypothesis generation, integration of multi-omics layers, and decision-making systems in clinical bioinformatics.
Machine Learning in Omics: Detecting Patterns That Matter
Machine learning, a subset of AI, has become a cornerstone in omics analysis. It involves training algorithms to recognize patterns in data, either with supervision (using labeled data) or without (unsupervised learning).
-
In genomics, ML models help predict the functional impact of mutations, detect regulatory elements, and identify genomic markers associated with diseases.
-
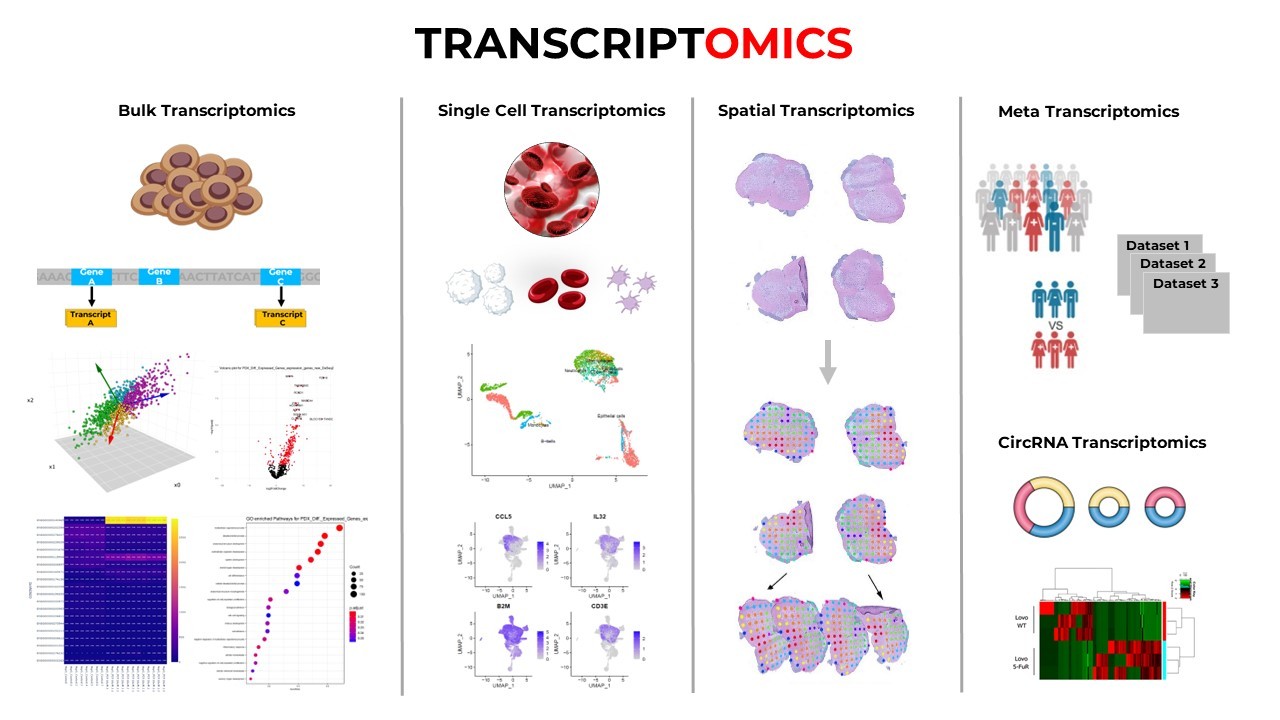
In transcriptomics, they are used to classify disease states based on gene expression profiles, cluster samples, and predict differentially expressed genes.
-
In proteomics, ML aids in identifying disease-specific protein expression patterns and classifying protein functions.
-
In metabolomics, ML helps in biomarker discovery and classifying metabolic profiles linked to disease states.
-
In epigenomics, ML is used for predicting chromatin states, methylation changes, and their influence on gene regulation.
Kourou et al. (2015) demonstrated the power of ML in cancer prediction, where algorithms like support vector machines and random forests outperformed conventional statistical models in predicting patient outcomes from omics data (https://doi.org/10.1016/j.jbi.2015.01.007).
Deep Learning: Peering Deeper into Biological Complexity
Deep learning builds on machine learning by using neural networks to model complex relationships in data. These models are especially powerful for large-scale and high-dimensional data, which makes them well-suited for omics applications.
-
In genomics, deep learning models like convolutional neural networks (CNNs) have been used to identify promoter regions, enhancer elements, and splicing sites directly from DNA sequences.
-
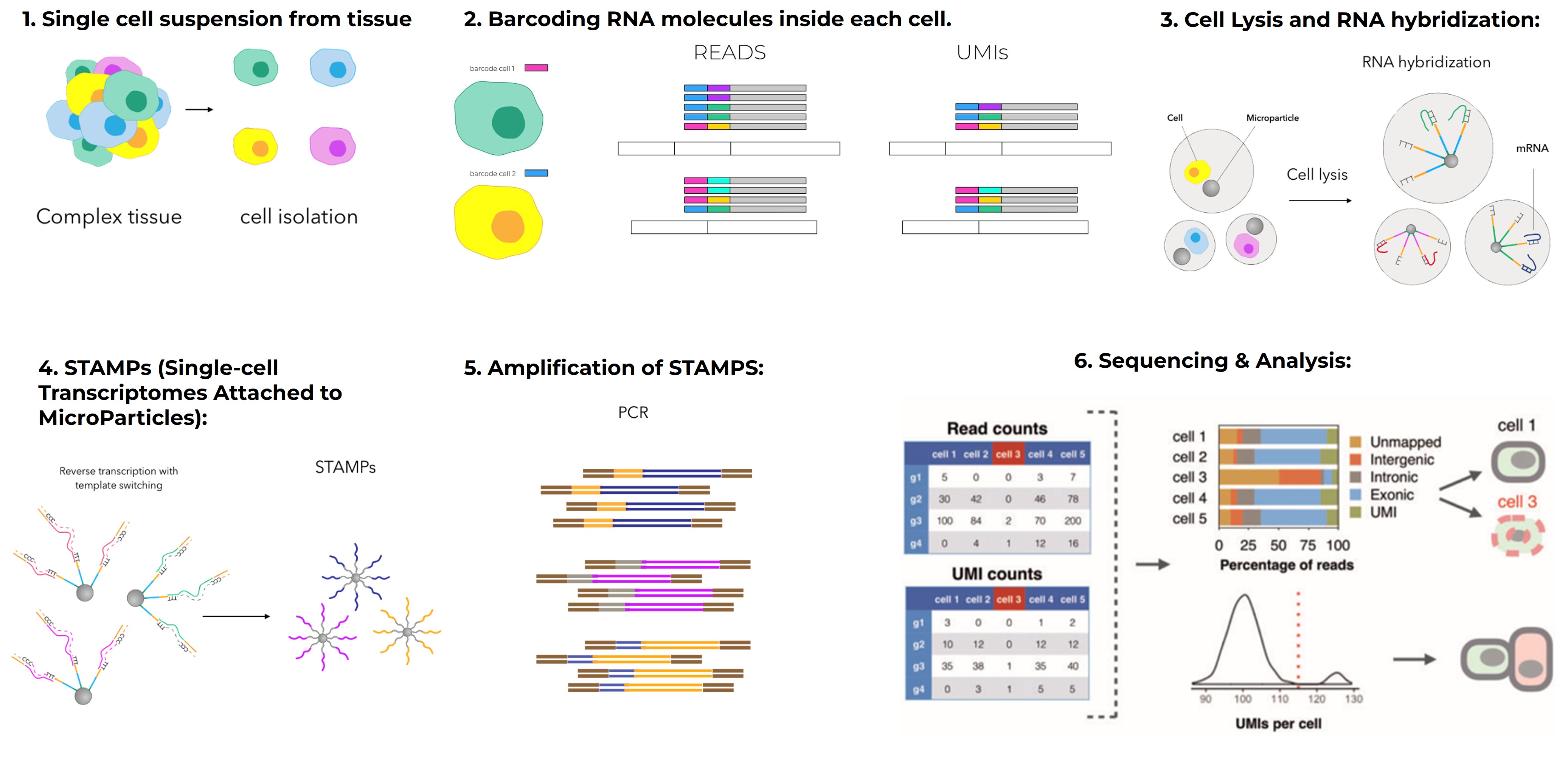
In single-cell transcriptomics, models such as variational autoencoders (VAEs) help denoise noisy datasets, detect rare cell types, and infer cell lineages.
-
In proteomics, DL helps predict protein structures and interactions.
-
In epigenomics, models like DeepSEA predict chromatin accessibility and regulatory element impact.
A notable example is the DeepSEA model developed by Zhou and Troyanskaya in 2015, which accurately predicted the chromatin effects of noncoding genetic variants (https://www.nature.com/articles/nmeth.3547).
Natural Language Processing: Mining Knowledge from Literature
NLP, another subfield of AI, allows machines to read and understand human language. In omics, NLP plays a key role in mining biomedical literature to extract gene–disease associations, drug–target interactions, and functional annotations.
-
In genomics and transcriptomics, NLP tools extract biological pathways, regulatory relationships, and variant interpretations from literature.
-
In clinical omics, NLP links EMR (electronic medical record) data with genomic profiles for clinical decision-making.
Tools like BioBERT, SciSpacy, and PubTator are designed to scan millions of abstracts and full-text articles, retrieving relevant biological relationships at scale. These models can be integrated into multi-modal pipelines that combine omics and textual data for better insight.
Generative AI: Synthesizing Novel Biological Insights
Generative AI, a subset of DL, creates new data or designs based on learned patterns. In omics, generative models have opened new frontiers:
-
In rare disease genomics, generative models create synthetic datasets to augment small sample sizes.
-
In protein informatics, models like AlphaFold and GAN-based approaches predict novel protein structures.
-
In drug discovery, these models propose new drug-like molecules or predict drug–target binding based on omics signatures.
LLMs: Large Language Models in Omics Intelligence
LLMs like GPT-4 combine capabilities of NLP, generative AI, and deep learning. They are revolutionizing omics research through:
-
Literature summarization for any given gene or disease
-
Contextual linking of omics datasets with biomedical evidence
-
Suggesting gene candidates for functional studies
-
Extracting therapeutic targets by mining across millions of full-text scientific documents
Integrated AI Pipelines in Practice
Today, omics analysis pipelines increasingly incorporate AI from end to end. For example, in a study on breast cancer, researchers may start by extracting features from genomic, transcriptomic, and proteomic datasets. These are then filtered and selected using random forest or LASSO regression. Deep learning models are applied to build predictive tools, while NLP is used to validate results with evidence from literature.
Such pipelines have led to breakthroughs in cancer subtype classification, biomarker discovery, and therapeutic response prediction. These aren’t just academic exercises-they’re informing clinical trials and treatment plans.
A Timeline of AI’s Rise in Omics
2001: Bioconductor project launched, bringing R-based tools to genomics.
2012: Deep learning resurgence begins; spillover into biology begins.
2015: DeepSEA introduces deep learning to functional genomics.
2017: scVI offers generative deep learning models for single-cell RNA-seq.
2019: BioBERT and similar models bring transformer-based NLP to biomedical texts.
2023: and beyond: Multi-modal foundation models trained on omics and clinical data emerge.
Learning AI for Omics: Where to Start
You don’t need to be a computer scientist to apply AI to omics research. Python is the most popular language for building AI workflows. Libraries such as scikit-learn (https://scikit-learn.org/), TensorFlow (https://www.tensorflow.org/), and PyTorch (https://pytorch.org/) are widely used. For NLP, tools like BioBERT (https://github.com/dmis-lab/biobert) are freely available.
The LBRN Training Platform provides beginner-friendly, application-focused training in AI and omics. With real datasets and structured exercises, students can learn to build models that classify disease states, predict gene–drug interactions, or identify novel cell types.
Conclusion: AI Is Reshaping Biology-And You Can Be Part of It
AI is more than a buzzword in life sciences-it’s the next big leap in how we understand biology. Whether you’re studying how genes cause disease, how cells respond to drugs, or how to design a personalized therapy, AI offers the tools to transform data into discovery.
For students, AI in omics is a field where biology meets computation in the most exciting ways. With accessible learning resources, open datasets, and growing demand in research and industry, there’s never been a better time to start. Biology has entered the age of intelligent systems-and every future biologist needs to speak the language of algorithms.